Author: Fairlie Reese
Data download and formatting
Long-read data
Note:
- Astrocyte data currently not publically available. This page will be updated when it is!
- These files already contain full-length non-chimeric reads. They have been processed as per the pipeline outlined here
# download files
wget http://crick.bio.uci.edu/freese/210413_pgp1_hub/encode_files_dl.txt .
xargs -L 1 curl -O -J -L < files.txt
# unzip
gunzip *fastq.gz
# rename them to make sense
mv ENCFF954UFG.fastq > pgp1_1.fastq
mv ENCFF251CBB.fastq > pgp1_2.fastq
mv ENCFF919JFJ.fastq > excite_neuron_1.fastq
mv ENCFF982WKN.fastq > excite_neuron_2.fastq
# mv ____.fastq > astro_1.fastq
# mv ____.fastq > astro_2.fastq
Reference data
# human reference genome
wget https://www.encodeproject.org/files/GRCh38_no_alt_analysis_set_GCA_000001405.15/@@download/GRCh38_no_alt_analysis_set_GCA_000001405.15.fasta.gz
gunzip GRCh38_no_alt_analysis_set_GCA_000001405.15.fasta.gz
mv GRCh38_no_alt_analysis_set_GCA_000001405.15.fasta hg38.fasta
# human reference genome chrom sizes
wget https://hgdownload-test.gi.ucsc.edu/goldenPath/hg38/bigZips/hg38.chrom.sizes .
# gencode v29 human reference transcriptome
wget https://www.encodeproject.org/files/gencode.v29.primary_assembly.annotation_UCSC_names/@@download/gencode.v29.primary_assembly.annotation_UCSC_names.gtf.gz
gunzip gencode.v29.primary_assembly.annotation_UCSC_names.gtf.gz
mv gencode.v29.primary_assembly.annotation_UCSC_names.gtf gencode.v29.annotation.gtf
Set up a small file holding the names of each sample
Trust me it’s easier this way
touch "samples.txt"
printf "pgp1_1\n" >> samples.txt
printf "pgp1_2\n" >> samples.txt
printf "excite_neuron_1\n" >> samples.txt
printf "excite_neuron_2\n" >> samples.txt
printf "astro_1\n" >> samples.txt
printf "astro_2\n" >> samples.txt
Minimap
Map each set of reads to the reference genome. Meant to be run on the cluster.
module load samtools
module load minimap2/2.17
genome=hg38.fasta
# this will loop through and map each sample in samples.txt!
while read p
do
fastq=${p}.fastq
sam=${p}_mapped.sam
log=${p}_minimap.log
minimap2 \
-t 16 \
-ax splice:hq \
-uf \
--MD \
$genome \
$fastq > \
$sam 2> \
$log
done < samples.txt
TranscriptClean
Correct common long-read sequencing artifacts. Meant to be run on the cluster.
Download TranscriptClean from here: https://github.com/mortazavilab/TranscriptClean, and use the path where you downloaded it to as tc_path in the following block.
module load samtools
tc_path=/dfs6/pub/freese/mortazavi_lab/bin/TranscriptClean/
genome=hg38.fasta
while read p
do
sam=${p}_mapped.sam
bam=${p}_mapped.bam
sort_bam=${p}_sorted.bam
sort_sam=${p}_sorted.sam
# first sort the sam file
samtools view -Sb $sam > $bam
samtools sort $bam > $sort_bam
samtols view -h $sort_bam > $sort_sam
# run TranscriptClean
python ${tc_path}/TranscriptClean.py \
--sam $sort_sam \
--genome $genome \
-t 16 \
--canonOnly \
--deleteTmp \
--outprefix $p
done < samples.txt
TALON
TALON label reads
Before running TALON, we have to determine what the nucleotide composition of the end of each read is, which will help us filter for internal priming.
Download and install TALON according to the instructions on the TALON repository: https://github.com/mortazavilab/TALON
genome=hg38.fasta
while read p
do
sam=${p}_clean.sam
talon_label_reads \
--f $sam \
--g $genome \
--t 16 \
--ar 20 \
--deleteTmp \
--o $p
done < samples.txt
TALON database initialization
Before we run TALON on our reads, we have to add a reference annotation to compare it to
annot=gencode.v29.annotation.gtf
talon_initialize_database \
--f $annot \
--g hg38 \
--a gencode_v29 \
--l 0 \
--idprefix ENCODEH \
--5p 500 \
--3p 300 \
--o pgp1
Create a TALON config file
Create a comma-separated file that provides the sample name, sample description, platform, and location of each input sam file.
touch talon_config.csv
printf "pgp1_1,pgp1,SequelI,pgp1_1_labeled.sam\n" >> talon_config.csv
printf "pgp1_2,pgp1,SequelI,pgp1_2_labeled.sam\n" >> talon_config.csv
printf "excite_neuron_1,excitatory_neuron,SequelI,excite_neuron_1_labeled.sam\n" >> talon_config.csv
printf "excite_neuron_2,excitatory_neuron,SequelI,excite_neuron_2_labeled.sam\n" >> talon_config.csv
printf "astro_1,astrocyte,SequelII,astro_1_labeled.sam\n" >> talon_config.csv
printf "astro_2,astrocyte,SequelII,astro_2_labeled.sam\n" >> talon_config.csv
Run TALON
talon \
--f talon_config.csv \
--db pgp1.db \
--build hg38 \
--t 16 \
--o $pgp1
Filter output transcripts
Filter novel transcripts for internal priming and for reproducibility
db=pgp1.db
talon_filter_transcripts \
--db $db \
-a gencode_v29 \
--maxFracA=0.5 \
--minCount=5 \
--minDatasets=2 \
--o pgp1_pass_list.csv
Create an unfiltered abundance file
db=pgp1.db
talon_abundance \
--db $db \
-a gencode_v29 \
-b hg38 \
--o pgp1
Visualizing TALON output
import pandas as pd
import seaborn as sns
import matplotlib.pyplot as plt
import scanpy as sc
import numpy as np
import anndata
import scipy.stats as st
import statsmodels.stats as stm
from statsmodels.stats.multitest import multipletests
import swan_vis as swan
from matplotlib.colors import ListedColormap
import matplotlib as mpl
import os
import shlex
import subprocess
def get_talon_nov_colors():
c_dict = {'Known': '#009E73',
'ISM': '#0072B2',
'NIC': '#D55E00',
'NNC': '#E69F00',
'Antisense': '#000000',
'Intergenic': '#CC79A7',
'Genomic': '#F0E442'}
order = ['Known', 'ISM', 'NIC', 'NNC', 'Antisense', 'Intergenic', 'Genomic']
return c_dict, order
def add_perc(ax, data, feature):
total = data[feature].sum()
ylim = ax.get_ylim()[1]
for p in ax.patches:
percentage = '{:.1f}%'.format(100 * p.get_height()/total)
x = p.get_x() + p.get_width() / 2 - 0.4
y = p.get_y() + p.get_height() + ylim*0.00625
ax.annotate(percentage, (x, y), size = 14)
def plot_read_novelty(df, opref, c_dict, order,
ylim=None, title=None,
datasets='all'):
sns.set_context("paper", font_scale=1.6)
sns.set_style(style='white')
temp = df.copy(deep=True)
# filter on datasets
if datasets != 'all':
temp = temp.loc[temp.dataset.isin(datasets)]
# count number of reads per cat
temp = temp[['transcript_novelty', 'read_name']].groupby('transcript_novelty').count()
temp.reset_index(inplace=True)
temp.rename({'read_name':'counts'}, axis=1, inplace=True)
print(temp)
# actual plotting
g = sns.catplot(data=temp, x='transcript_novelty',
y='counts', kind='bar',
palette=c_dict, order=order)
[plt.setp(ax.get_xticklabels(), rotation=90) for ax in g.axes.flat]
g.set_ylabels('Reads')
g.set_xlabels('Transcript novelty')
# add percentage labels
ax = g.axes[0,0]
add_perc(ax, temp, 'counts')
if ylim:
g.set(ylim=(0,ylim))
# # add title
# if not title:
# g.fig.suptitle('Reads per novelty category')
# else:
# g.fig.suptitle('{} reads per novelty category'.format(title))
# save figure
fname = '{}_read_novelty'.format(opref)
g.savefig(fname+'.pdf', dpi=300)
def plot_transcript_novelty(df, oprefix, c_dict, order, \
ylim=None, title=None,
whitelist=None, datasets='all', save_type='pdf'):
sns.set_context('paper', font_scale=1.6)
sns.set_style(style='white')
temp = df.copy(deep=True)
# remove transcripts that are not on whitelist
if whitelist:
temp = temp.loc[temp.transcript_ID.isin(whitelist)]
# filter on datasets
if datasets != 'all':
temp = temp.loc[temp.dataset.isin(datasets)]
# count number of isoforms per cat
temp = temp[['transcript_ID', 'transcript_novelty', 'read_name']].groupby(['transcript_ID', 'transcript_novelty']).count()
temp.reset_index(inplace=True)
temp.drop('read_name', axis=1, inplace=True)
temp = temp.groupby('transcript_novelty').count()
temp.reset_index(inplace=True)
temp.rename({'transcript_ID': 'counts'}, axis=1, inplace=True)
print(temp)
# actual plotting
g = sns.catplot(data=temp, x='transcript_novelty',
y='counts', kind='bar',
palette=c_dict, order=order)
[plt.setp(ax.get_xticklabels(), rotation=90) for ax in g.axes.flat]
g.set_ylabels('Isoforms')
g.set_xlabels('Transcript novelty')
# add percentage labels
ax = g.axes[0,0]
add_perc(ax, temp, 'counts')
if ylim:
g.set(ylim=(0,ylim))
# # add title
# if not title:
# g.fig.suptitle('Transcript models per novelty category')
# else:
# g.fig.suptitle('{} transcript models per novelty category'.format(title))
# save figure
fname = '{}_isoform_novelty'.format(oprefix)
if save_type == 'png':
g.savefig(fname+'.png', dpi=300)
elif save_type == 'pdf':
g.savefig(fname+'.pdf', dpi=300)
plt.show()
plt.clf()
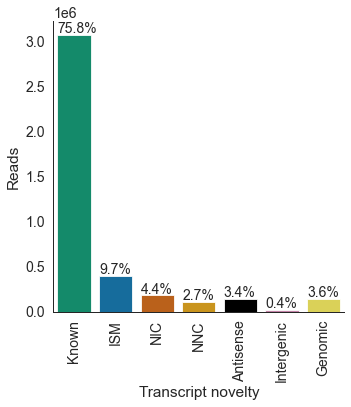
# number / proportion of reads per novelty category
df = pd.read_csv('pgp1_talon_read_annot.tsv', sep='\t')
c_dict, order = get_talon_nov_colors()
opref = 'pgp1'
plot_read_novelty(df, opref, c_dict, order,
ylim=None, title=None,
datasets='all')
transcript_novelty counts
0 Antisense 137767
1 Genomic 143842
2 ISM 393877
3 Intergenic 16599
4 Known 3068807
5 NIC 180012
6 NNC 109720
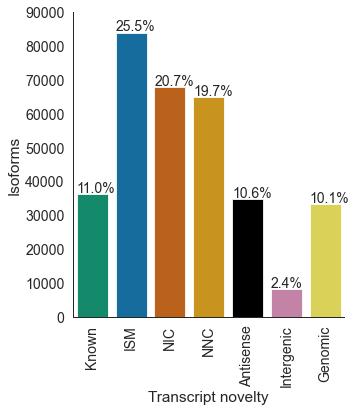
# unfiltered transcripts per novelty category
df = pd.read_csv('pgp1_talon_read_annot.tsv', sep='\t')
c_dict, order = get_talon_nov_colors()
datasets = ['pgp1_1', 'pgp1_2', 'excite_neuron_1', 'excite_neuron_2', 'astro_1', 'astro_2']
opref = 'pgp1'
plot_transcript_novelty(df, opref, c_dict, order, \
ylim=90000, title='Unfiltered', datasets='all', save_type='pdf')
transcript_novelty counts
0 Antisense 34773
1 Genomic 33150
2 ISM 83895
3 Intergenic 8044
4 Known 36205
5 NIC 67933
6 NNC 64771
<Figure size 432x288 with 0 Axes>
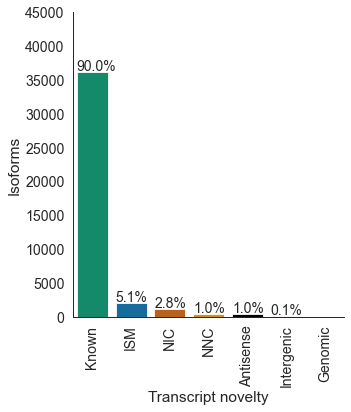
# filtered transcripts per novelty category
df = pd.read_csv('pgp1_talon_read_annot.tsv', sep='\t')
c_dict, order = get_talon_nov_colors()
datasets = ['pgp1_1', 'pgp1_2', 'excite_neuron_1', 'excite_neuron_2', 'astro_1', 'astro_2']
opref = 'pgp1'
pass_list = pd.read_csv('pgp1_pass_list.csv', header=None, names=['gene_ID', 'transcript_ID'])
pass_list = pass_list.transcript_ID.unique().tolist()
plot_transcript_novelty(df, opref, c_dict, order, \
ylim=45000, title='Filtered',
whitelist=pass_list, datasets='all', save_type='pdf')
transcript_novelty counts
0 Antisense 414
1 ISM 2058
2 Intergenic 32
3 Known 36205
4 NIC 1108
5 NNC 414
<Figure size 432x288 with 0 Axes>
df = pd.read_csv('pgp1_talon_read_annot.tsv', sep='\t')
datasets = ['pgp1_1', 'pgp1_2', 'excite_neuron_1', 'excite_neuron_2', 'astro_1', 'astro_2']
opref = 'pgp1'
pass_list = pd.read_csv('pgp1_pass_list.csv', header=None, names=['gene_ID', 'transcript_ID'])
pass_list = pass_list.transcript_ID.unique().tolist()
print('Number of reads before filtering: {}'.format(len(df.index)))
print('Number of reads after filtering: {}'.format(len(df.loc[df.transcript_ID.isin(pass_list)].index)))
Number of reads before filtering: 4050624
Number of reads after filtering: 3377394
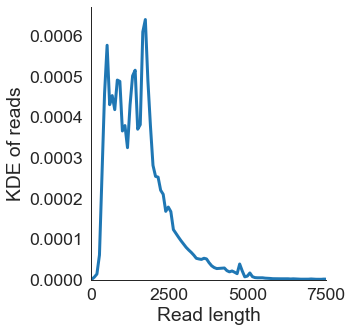
# Distribution of read lengths
df = pd.read_csv('pgp1_talon_read_annot.tsv', sep='\t')
sns.set_context('paper', font_scale=2)
ax = sns.displot(data=df, x='read_length', kind='kde', linewidth=3)
ax.set(xlabel='Read length', ylabel='KDE of reads',
title='', xlim=(0,7500),
xticks=[0, 2500, 5000, 7500])
plt.savefig('{}_read_length_kde.pdf'.format(opref), dpi=300, bbox_inches='tight')
TSS calling
Subset TSS reads
For TSSs, we’ll consider Known, prefix ISM, NIC, and NNC reads, as these are more likely to contain putative 5’ ends.
annot = 'pgp1_talon_read_annot.tsv'
df = pd.read_csv(annot, sep='\t')
tss_df = df.loc[df.transcript_novelty.isin(['Known', 'NIC', 'NNC', 'ISM'])]
tss_df = tss_df.loc[tss_df.ISM_subtype.isin(['None', 'Prefix', 'Both'])]
tss_reads = tss_df.read_name.tolist()
# tss
fname = 'tss_read_names.txt'
with open(fname, 'w') as ofile:
for r in tss_reads:
ofile.write(r+'\n')
This part should be run on the cluster or using bash. It relies on the existence of an intermediate merged bam file that TALON makes when it is running.
The picard_path variable refers to wherever Picard is installed.
module load samtools
picard_path=/opt/apps/picard-tools/1.87/
in_bam=talon_tmp/merged.bam
out_bam=talon_tmp/merged_rg.bam
tss_reads=tss_read_names.txt
tss_out=tss_reads.bam
# first add the RG header tags for each RG
rg_tags="pgp1_1 pgp1_2 excite_neuron_1 excite_neuron_2 astro_1 astro_2"
samtools view -H $in_bam > header.sam
for tag in $rg_tags
do
printf '@RG\tID:${tag}\tPL:PacBio\tSM:PGP1\n' >> header.sam
done
samtools reheader header.sam $in_bam > $out_bam
# then limit to the known, prefix ISM, NIC, and NNC reads
java -jar ${picard_path}FilterSamReads.jar \
I=$out_bam \
O=$tss_out \
READ_LIST_FILE=$tss_reads \
FILTER=includeReadList \
CREATE_INDEX=true
# index bam file
samtools index $tss_out
Call TSSs
Call transcription start sites using a TSS caller that, roughly, calls peaks on read starts from long read data.
You can download the tool from here: https://github.com/ENCODE-AWG/tss-annotation. Your tss_dir should be wherever you install it.
tss_dir=~/mortazavi_lab/bin/tss-annotation/long_read/
python ${tss_dir}pacbio_to_tss.py \
-i tss_reads.bam \
--window-size=50 \
--expression-threshold=2 \
-o unfilt_tss.bed \
-r \
-n rev_tss.bw \
-p fwd_tss.bw
Get the read names associated with each TSS peak so that we can associate TSSs with genes
merged_bam=talon_tmp/merged.bam
python ${tss_dir}tss_reads.py \
-i $merged.bam \
-r unfilt_tss.bed \
-o tss_reads.bed
Filter TSSs based on number of reads and expression within gene
Require TSSs to have at least 2 supporting reads and at least 10 percent of the number of reads that the top-expressed TSS in the gene has.
Additionally save TSS expression in a counts matrix.
# filter a list of TSSs for each gene
annot = 'pgp1_talon_read_annot.tsv'
tss_reads = 'tss_reads.bed'
df = pd.read_csv(annot, sep='\t')
# remove sirvs and erccs
df = df.loc[~df.chrom.str.contains('SIRV')]
df = df.loc[~df.chrom.str.contains('ERCC')]
ends = pd.read_csv(tss_reads, sep='\t', header=None,
names=['chrom', 'start', 'end', 'read_name', 'tss_id', 'strand'])
# merge on read name
df = df.merge(ends, how='inner', on='read_name')
# groupby on gene and tss
df = df[['read_name', 'annot_gene_name', 'tss_id']].groupby(['annot_gene_name', 'tss_id']).count()
# filter tsss for those that have >10% of the reads
# for the most highly-expressed tss of the gene
df.reset_index(inplace=True)
df.rename({'read_name':'count'}, axis=1, inplace=True)
temp = df.loc[df.apply(lambda x: x['count'] >= df.loc[df.annot_gene_name==x.annot_gene_name, 'count'].max()*0.1, axis=1)]
temp.loc[temp.annot_gene_name == 'MBP']
# assign each TSS a name, and quantify TSS exp
temp.sort_values(by=['annot_gene_name'], inplace=True)
temp['tss_id_2'] = np.nan
prev_gene = None
for ind, entry in temp.iterrows():
curr_gene = entry.annot_gene_name
if curr_gene != prev_gene:
i = 1
else:
i += 1
prev_gene = curr_gene
temp.loc[ind, 'tss_id_2'] = '{}_{}'.format(curr_gene, i)
# merged called TSSs back in with read annot
df = pd.read_csv(annot, sep='\t')
ends = pd.read_csv(tss_reads, sep='\t', header=None,
names=['chrom', 'start', 'end', 'read_name', 'tss_id', 'strand'])
ends = ends[['read_name', 'tss_id']]
# dump filtered TSSs to bed
fname = 'unfilt_tss.bed'
end_regions = pd.read_csv(fname, sep='\t', header=None,
names=['chrom', 'start', 'end', 'tss_id', 'read_count', 'strand',
'sth1', 'sth2', 'sth3', 'sth4', 'sth5'])
filt_ends = end_regions.merge(temp[['tss_id', 'tss_id_2']], how='inner', on='tss_id')
filt_ends['score'] = 0
filt_ends = filt_ends[['chrom', 'start', 'end', 'tss_id_2', 'score', 'strand']]
fname = 'filt_tss.bed'
filt_ends.to_csv(fname, sep='\t', header=None, index=False)
# merge on read name
df = df.merge(ends, how='inner', on='read_name')
temp = temp[['annot_gene_name', 'tss_id', 'tss_id_2']]
df = df.merge(temp, how='inner', on=['annot_gene_name', 'tss_id'])
# format like talon ab
cols = ['annot_gene_name', 'annot_gene_id', 'dataset', 'tss_id_2']
df = df[cols+['read_name']].groupby(cols).count().reset_index()
df.rename({'read_name':'counts', 'tss_id_2':'tss_id'}, axis=1, inplace=True)
df = df.pivot(index=['annot_gene_name', 'annot_gene_id', 'tss_id'], columns='dataset', values='counts')
df.reset_index(inplace=True)
df = df.rename_axis(None, axis=1)
df.fillna(0, inplace=True)
# i guess sum over genes with the same name smh
df.drop('annot_gene_id', axis=1, inplace=True)
df = df.groupby(['annot_gene_name', 'tss_id']).sum().reset_index()
df.to_csv('pgp1_tss_talon_abundance.tsv', sep='\t', index=False)
/Users/fairliereese/miniconda3/lib/python3.7/site-packages/ipykernel_launcher.py:29: SettingWithCopyWarning:
A value is trying to be set on a copy of a slice from a DataFrame
See the caveats in the documentation: https://pandas.pydata.org/pandas-docs/stable/user_guide/indexing.html#returning-a-view-versus-a-copy
/Users/fairliereese/miniconda3/lib/python3.7/site-packages/ipykernel_launcher.py:30: SettingWithCopyWarning:
A value is trying to be set on a copy of a slice from a DataFrame.
Try using .loc[row_indexer,col_indexer] = value instead
See the caveats in the documentation: https://pandas.pydata.org/pandas-docs/stable/user_guide/indexing.html#returning-a-view-versus-a-copy
/Users/fairliereese/miniconda3/lib/python3.7/site-packages/pandas/core/indexing.py:1765: SettingWithCopyWarning:
A value is trying to be set on a copy of a slice from a DataFrame.
Try using .loc[row_indexer,col_indexer] = value instead
See the caveats in the documentation: https://pandas.pydata.org/pandas-docs/stable/user_guide/indexing.html#returning-a-view-versus-a-copy
isetter(loc, value)
Swan
annot = 'gencode.v29.annotation.gtf'
# create a config file
df = pd.read_csv('samples.txt', header=None, names=['col'])
df['fname'] = 'pgp1.db'
df['counts_file'] = 'pgp1_talon_abundance.tsv'
df['count_cols'] = df['col']
df['tid_col'] = 'annot_transcript_id'
df['dataset_name'] = df['col']
df['whitelist'] = 'pgp1_pass_list.csv'
df.to_csv('swan_config.tsv', sep='\t', index=False)
# initialize SwanGraph
sg = swan.SwanGraph()
# add annotation GTF
sg.add_annotation(annot)
# add datasets and save graph
sg.add_datasets('swan_config.tsv')
sg.save_graph('swan')
Adding dataset annotation to the SwanGraph
Adding dataset pgp1_1 to the SwanGraph
Adding dataset pgp1_2 to the SwanGraph
Adding dataset excite_neuron_1 to the SwanGraph
Adding dataset excite_neuron_2 to the SwanGraph
Adding dataset astro_1 to the SwanGraph
Adding dataset astro_2 to the SwanGraph
Saving graph as swan.p
# detect IR and ES
g_es, t_es, e_es = sg.find_es_genes()
g_ir, t_ir, e_ir = sg.find_ir_genes()
Analyzing 866 intronic edges for ES
Found 237 novel es events in 275 transcripts.
Analyzing 2411 exonic edges for IR
Found 71 novel ir events from 82 transcripts.
# plot gene summary/transcript path graph for a gene with IR (SARS)
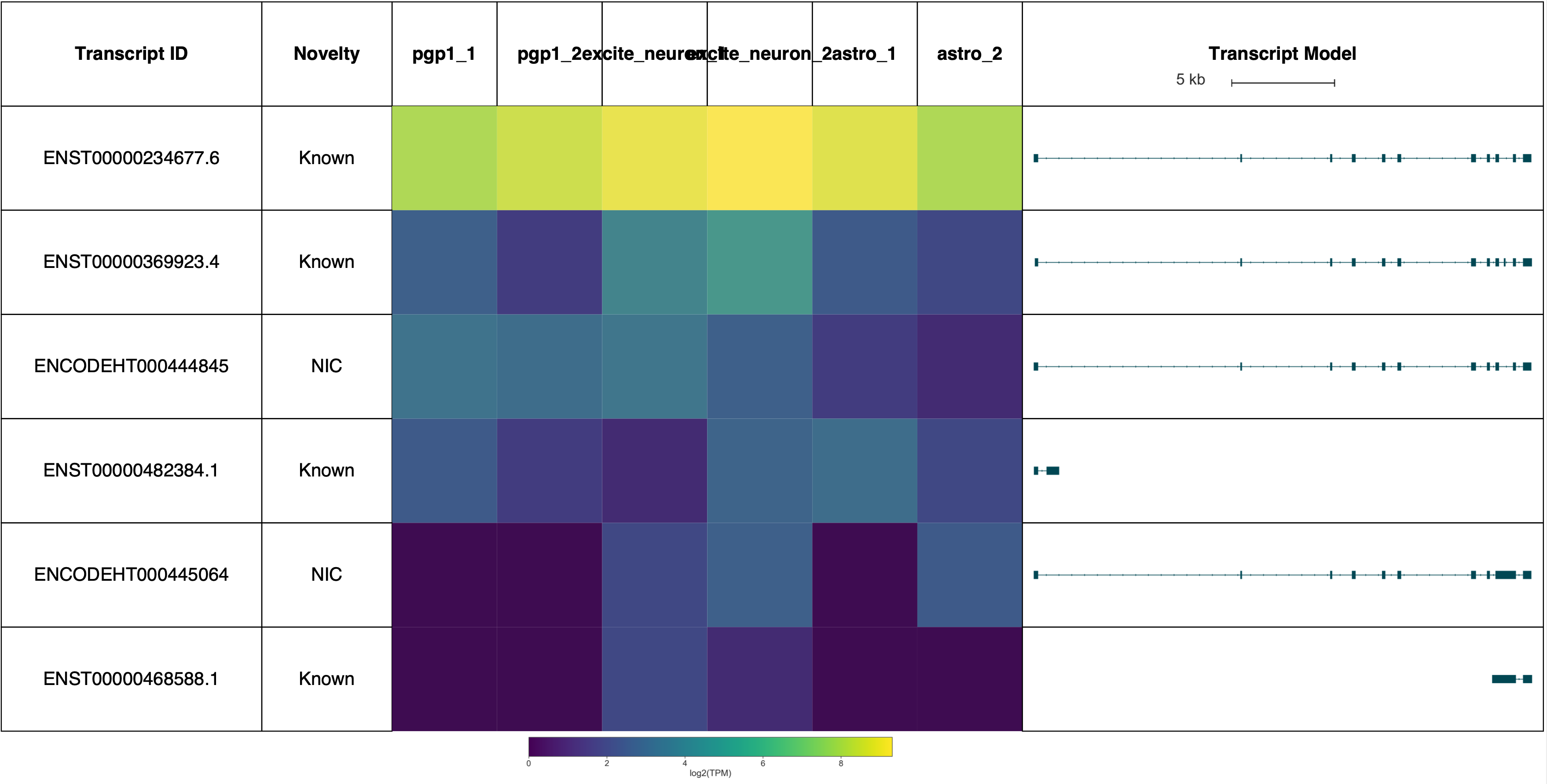
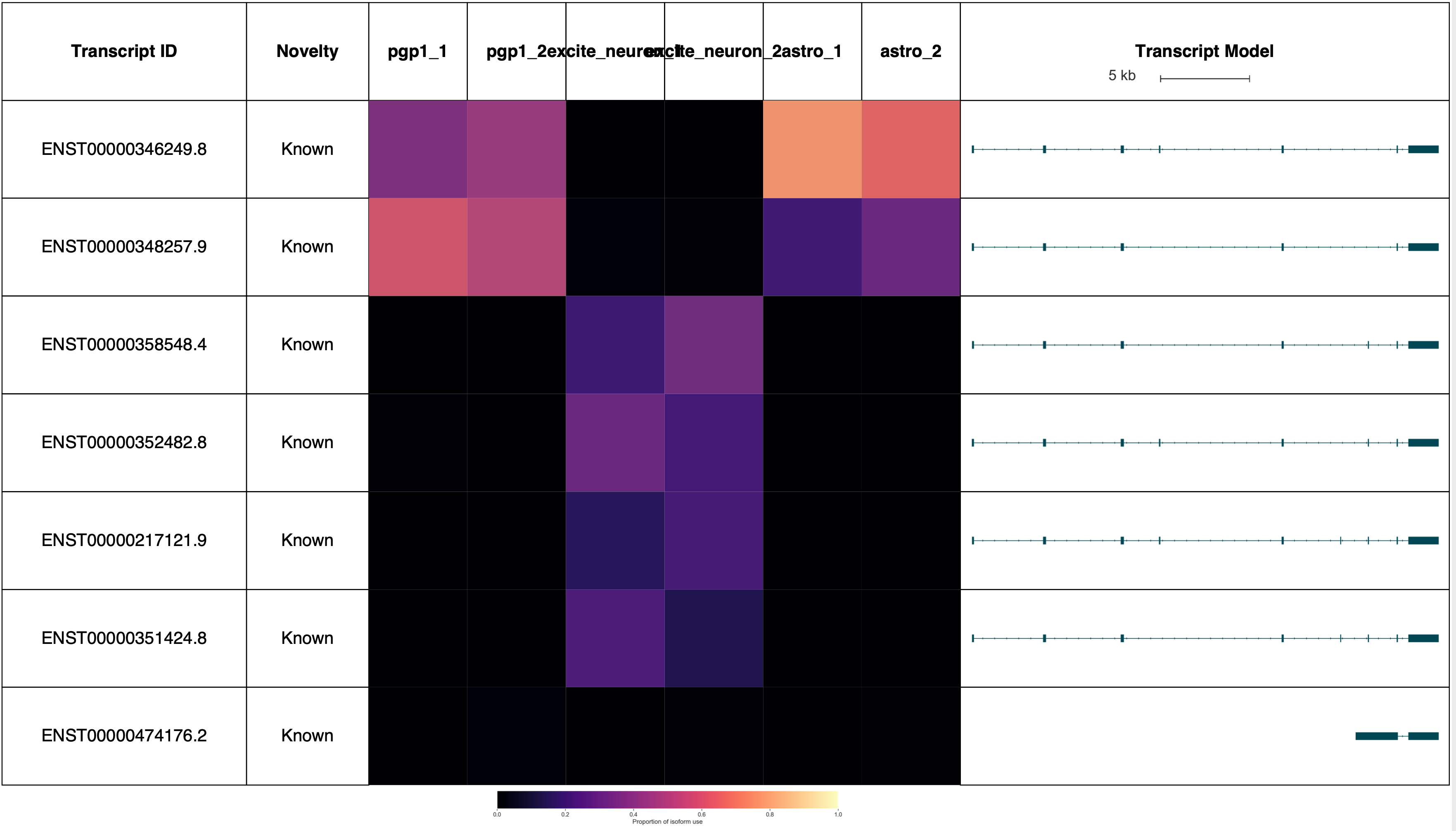
sg.plot_graph('SARS', indicate_novel=True, display=True, prefix='figures/SARS')
sg.plot_transcript_path('ENCODEHT000445064', indicate_novel=True, display=True, prefix='figures/SARS')
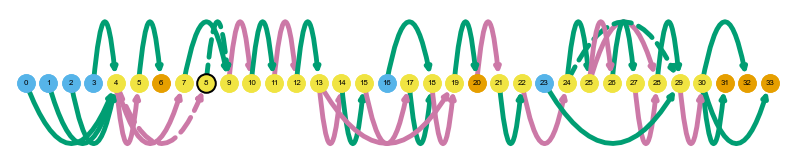
Saving summary graph for ENSG00000031698.12 as figures/SARS_novel_ENSG00000031698.12_summary.png
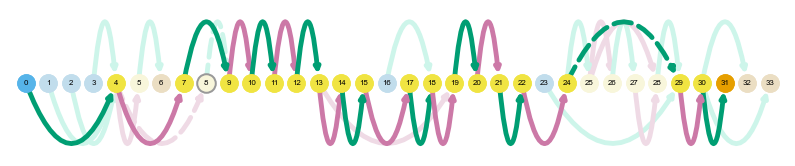
Saving transcript path graph for ENCODEHT000445064 as figures/SARS_novel_ENCODEHT000445064_path.png
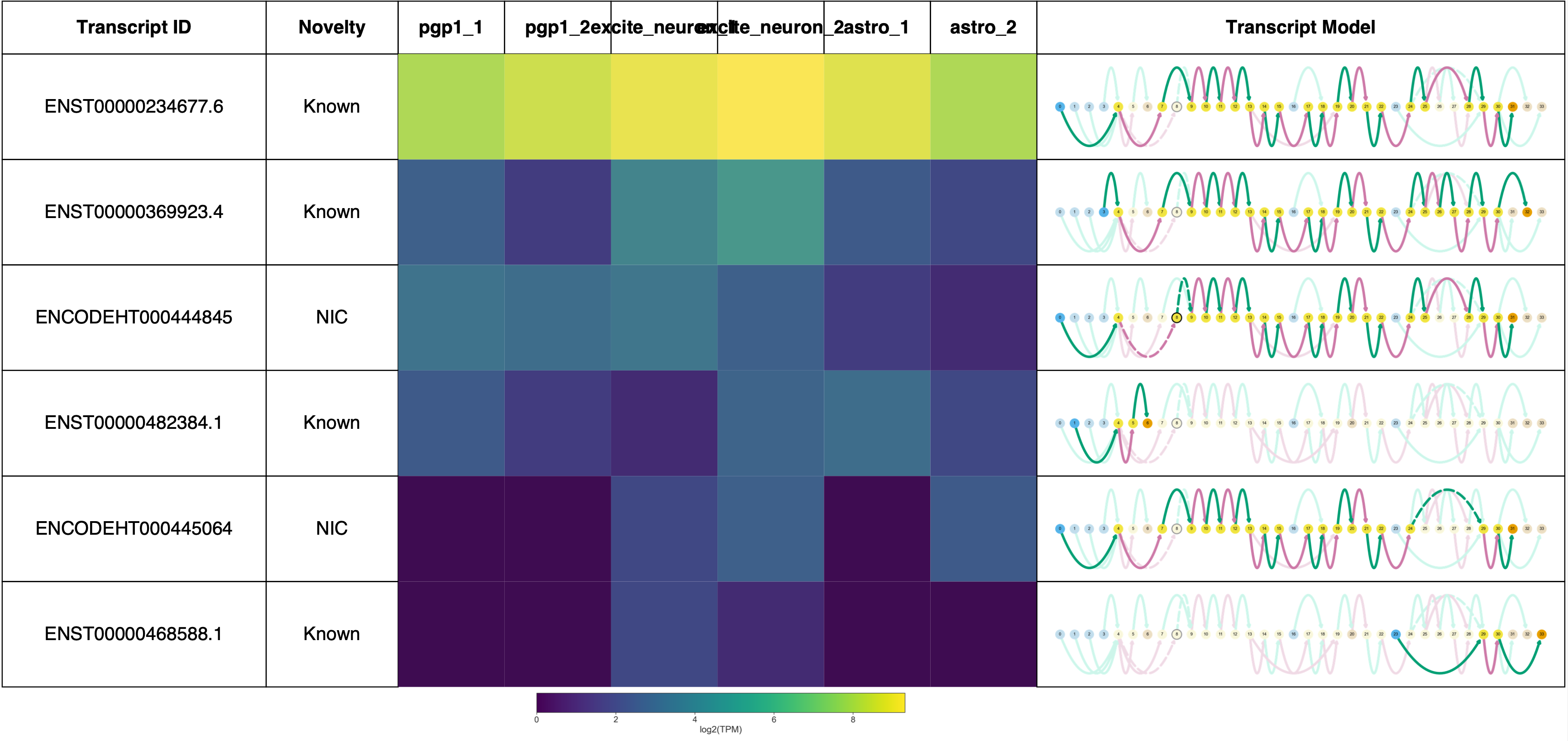
# plot gene report for the same gene with IR (SARS)
gname = 'SARS'
sg.gen_report(gname, prefix='figures/{}'.format(gname),
heatmap=True,
cmap='viridis',
indicate_novel=True,
novelty=True)
sg.gen_report(gname, prefix='figures/{}'.format(gname),
heatmap=True,
cmap='viridis',
browser=True,
novelty=True)
Plotting transcripts for ENSG00000031698.12
Saving transcript path graph for ENST00000234677.6 as figures/SARS_novel_ENST00000234677.6_path.png
Saving transcript path graph for ENST00000369923.4 as figures/SARS_novel_ENST00000369923.4_path.png
Saving transcript path graph for ENCODEHT000444845 as figures/SARS_novel_ENCODEHT000444845_path.png
Saving transcript path graph for ENST00000482384.1 as figures/SARS_novel_ENST00000482384.1_path.png
Saving transcript path graph for ENCODEHT000445064 as figures/SARS_novel_ENCODEHT000445064_path.png
Saving transcript path graph for ENST00000468588.1 as figures/SARS_novel_ENST00000468588.1_path.png
Generating report for ENSG00000031698.12
Plotting transcripts for ENSG00000031698.12
Saving transcript path graph for ENST00000234677.6 as figures/SARS_browser_ENST00000234677.6_path.png
Saving transcript path graph for ENST00000369923.4 as figures/SARS_browser_ENST00000369923.4_path.png
Saving transcript path graph for ENCODEHT000444845 as figures/SARS_browser_ENCODEHT000444845_path.png
Saving transcript path graph for ENST00000482384.1 as figures/SARS_browser_ENST00000482384.1_path.png
Saving transcript path graph for ENCODEHT000445064 as figures/SARS_browser_ENCODEHT000445064_path.png
Saving transcript path graph for ENST00000468588.1 as figures/SARS_browser_ENST00000468588.1_path.png
Generating report for ENSG00000031698.12
(Forgive the terrible sample labels, this will be fixed in a future Swan release!)
TSS and isoform switching
Detect statistically-significant instances of isoform or TSS switching from the TALON isoforms or from the called TSSs respectively.
# df: talon abundance file (either tss or transcript)
# cond_map: dictionary of {condition: [dataset1, dataset2]}; how you want to group datasets
# how: whether to make a tss or iso level adata; 'tss' or 'iso'
# pass_list: if 'iso', file of valid transcript IDs that pass filtering
def make_adata(df, cond_map, how='iso', pass_list=None):
# filter talon ab file based on pass list
# df = pd.read_csv(ab_file, sep='\t')
if pass_list:
pass_list = pd.read_csv(pass_list, header=None, names=['gene_id', 'transcript_id'])
df = df.loc[df.transcript_ID.isin(pass_list.transcript_id.tolist())]
# obs table
obs = pd.DataFrame.from_dict(cond_map, orient='index')
obs.reset_index(inplace=True)
id_vars = ['index']
value_vars = obs.columns[1:]
obs = obs.melt(id_vars=id_vars, value_vars=value_vars)
obs.drop('variable', axis=1, inplace=True)
obs.rename({'index':'condition', 'value':'dataset'}, axis=1, inplace=True)
# var table
if how=='iso':
var_cols = ['annot_transcript_name', 'annot_gene_name',\
'annot_transcript_id', 'annot_gene_id', \
'gene_ID', 'transcript_ID', 'transcript_novelty', \
'ISM_subtype']
var = df[var_cols]
var.rename({'transcript_ID':'transcript_id', \
'gene_ID':'gene_id',\
'annot_gene_name': 'gene_name'}, axis=1, inplace=True)
if how=='tss':
var_cols = ['annot_gene_name', 'tss_id']
var = df[var_cols]
var.rename({'annot_gene_name': 'gene_name'}, axis=1, inplace=True)
# X table
df = df.transpose()
df = df.loc[df.index.isin(obs.dataset.tolist())]
obs_order = obs['dataset'].reset_index().set_index('dataset')
df['dataset_num'] = df.index.map(obs_order['index'])
df.sort_values('dataset_num', inplace=True)
df.drop('dataset_num', axis=1, inplace=True)
X = df.to_numpy()
adata = anndata.AnnData(obs=obs, var=var, X=X)
return adata
# gene_df: pandas dataframe with expression values in each condition for each TSS in a gene
# conditions: list of str of condition names
# rc: threshold of read count per gene in each condition necessary to test this gene
def test_gene(gene_df, conditions, col, id_col, rc=10):
gene_df = gene_df.pivot(index=col, columns=id_col, values='counts')
gene_df = gene_df.transpose()
groups = gene_df.columns.tolist()
gene_df['total_counts'] = gene_df[groups].sum(axis=1)
gene_df.sort_values(by='total_counts', ascending=False, inplace=True)
if len(gene_df.index) > 11:
gene_df.reset_index(inplace=True)
beep = gene_df.iloc[10:].sum()
beep[id_col] = 'all_other'
beep.index.name = None
beep = pd.DataFrame(beep).transpose()
gene_df = gene_df.iloc[:10]
gene_df = pd.concat([gene_df, beep])
# limit to just isoforms with > 0 expression in both conditions
cond1 = conditions[0]
cond2 = conditions[1]
gene_df = gene_df.loc[(gene_df[cond1]>0)&(gene_df[cond2]>0)]
# does this gene reach the desired read count threshold?
for cond in conditions:
if gene_df[cond].sum() < rc:
return np.nan, np.nan
# only do the rest if there's nothing left
if gene_df.empty:
return np.nan, np.nan
# calculate the percent of each sample each TSS accounts for
cond_pis = []
for cond in conditions:
total_col = '{}_total'.format(cond)
pi_col = '{}_pi'.format(cond)
total_count = gene_df[cond].sum()
cond_pis.append(pi_col)
gene_df[total_col] = total_count
gene_df[pi_col] = (gene_df[cond]/gene_df[total_col])*100
# compute isoform-level and gene-level delta pis
gene_df['dpi'] = gene_df[cond_pis[0]] - gene_df[cond_pis[1]]
gene_df['abs_dpi'] = gene_df.dpi.abs()
gene_dpi = gene_df.iloc[:2].abs_dpi.sum()
# chi squared test
chi_table = gene_df[conditions].to_numpy()
chi2, p, dof, exp = st.chi2_contingency(chi_table)
return p, gene_dpi
def filter_die_results(df, p, dpi):
df = df.loc[(df.adj_p_val<=p)&(df.dpi>=dpi)]
return df
# adata: adata with TSS or iso expression
# conditions: len 2 list of strings of conditions to compare
# col: string, which column the condition labels are in
# how: 'tss' or 'iso'
def get_die(adata, conditions, how='tss', rc=15):
if how == 'tss':
id_col = 'tss_id'
elif how == 'iso':
id_col = 'transcript_id'
# make df that we can groupby
col = 'condition'
colnames = adata.var[id_col].tolist()
rownames = adata.obs.dataset.tolist()
raw = adata.X
df = pd.DataFrame(data=raw, index=rownames, columns=colnames)
df.reset_index(inplace=True)
df.rename({'index':'dataset'}, axis=1, inplace=True)
samp = adata.obs[['dataset', col]]
df = df.merge(samp, how='left', on='dataset')
# limit to only the samples that we want in this condition
df[col] = df[col].astype('str')
df = df.loc[df[col].isin(conditions)]
# groupby sample type and sum over gen
df.drop('dataset', axis=1, inplace=True)
df = df.groupby(col).sum().reset_index()
# melty boi
tss_cols = df.columns.tolist()[1:]
df = df.melt(id_vars=col, value_vars=tss_cols)
# rename some cols
df.rename({'variable':id_col,'value':'counts'}, axis=1, inplace=True)
# merge with gene names
df = df.merge(adata.var, how='left', on=id_col)
# get total number of tss or iso / gene
bop = df[['gene_name', id_col]].groupby('gene_name').count().reset_index()
# construct tables for each gene and test!
gene_names = df.gene_name.unique().tolist()
gene_de_df = pd.DataFrame(index=gene_names, columns=['p_val', 'dpi'], data=[[np.nan for i in range(2)] for j in range(len(gene_names))])
for gene in gene_names:
gene_df = df.loc[df.gene_name==gene]
p, dpi = test_gene(gene_df, conditions, col, id_col, rc=rc)
gene_de_df.loc[gene, 'p_val'] = p
gene_de_df.loc[gene, 'dpi'] = dpi
# correct p values
gene_de_df.dropna(axis=0, inplace=True)
p_vals = gene_de_df.p_val.tolist()
_, adj_p_vals, _, _ = multipletests(p_vals, method='fdr_bh')
gene_de_df['adj_p_val'] = adj_p_vals
gene_de_df.reset_index(inplace=True)
return gene_de_df
def get_sample_colors():
c_dict = {'astro_1': '#f6ef7c', 'astro_2': '#eabc68',\
'excite_neuron_1': '#e4d3cd', 'excite_neuron_2': '#d3a8b2',\
'pgp1_1': '#bef4ff', 'pgp1_2': '#73a8b2'}
order = ['pgp1_1', 'pgp1_2', 'astro_1', 'astro_2', 'excite_neuron_1', 'excite_neuron_2']
return c_dict, order
def make_cond_map(groups, group_names):
cond_map = dict()
for group, group_name in zip(groups, group_names):
cond_map[group] = group_name
return cond_map
# calculate the normalized average or sum of TSS expression
# per cell from the TSS anndata object
def calc_exp(adata, groups, group_names, how='tss', cpm=False):
try:
adata.var.reset_index(inplace=True)
except:
pass
if how == 'tss':
id_col = 'tss_id'
elif how == 'iso':
id_col = 'transcript_id'
# conditions map
cond_map = make_cond_map(groups, group_names)
# print(cond_map)
col = 'condition'
adata.obs.rename({'dataset':'condition'}, axis=1, inplace=True)
# adata.obs[col] = adata.obs.dataset.map(cond_map)
# make df that we can groupby
colnames = adata.var[id_col].tolist()
rownames = adata.obs[col].tolist()
raw = adata.X
gene_names = adata.var.gene_name.tolist()
df = pd.DataFrame(data=raw, index=rownames, columns=colnames)
df.reset_index(inplace=True)
df.rename({'index':col}, axis=1, inplace=True)
samp = adata.obs[[col]]
df = df.merge(samp, how='left', on=col)
# limit to only the cells that we want in this condition
df[col] = df[col].astype('str')
df = df.loc[df[col].isin(group_names)]
# groupby sample type and sum over gen
# df.drop(col, axis=1, inplace=True)
df = df.groupby(col).sum().reset_index()
if cpm:
# since these values haven't been normalized yet, do that
# CPM : (counts/total_counts)* 1**6
# Note : ATAC values were pre-normalized
df.set_index(col, inplace=True)
df = df.transpose()
for c in group_names:
total_counts = df[c].sum()
df[c] = (df[c]/total_counts)*(1^6)
df = df.transpose()
df.reset_index(inplace=True)
# melty boi
tss_cols = df.columns.tolist()[1:]
df = df.melt(id_vars=col, value_vars=tss_cols)
# rename some cols
df.rename({'variable':id_col,'value':'counts'}, axis=1, inplace=True)
# add gene name
if how == 'tss':
temp = adata.var[[id_col, 'gene_name']]
df = df.merge(temp, how='left', on=id_col)
return df
def plot_tss_heatmap(adata, groups, group_names, gname, opref):
# calculate TSS expression per condition
adata.obs.drop('condition', axis=1, inplace=True)
tss_df = calc_exp(adata, groups, group_names, how='tss')
# subset by gene and calculate DPI per gene
tss_df = tss_df.loc[tss_df.gene_name == gname]
n_tss = len(tss_df.tss_id.unique())
tss_df.drop(['gene_name'], axis=1, inplace=True)
tss_df = tss_df.pivot(index='tss_id', columns='condition', values='counts')
tss_df = tss_df.div(tss_df.sum(axis=0), axis=1)
# get categorical colormap
tss_df = tss_df[groups]
mini_obs, cat_cmap = get_cat_cmap(groups)
# plot the figure
sns.set(rc={'figure.figsize':(12,7)})
sns.set_context('paper', font_scale=1.5)
fig = plt.figure()
# complicated subplot stuff
tss_ax = plt.subplot2grid((n_tss+1,1), loc=(0,0), rowspan=n_tss)
# fig, axes = plt.subplots(nrows=4)
fig.subplots_adjust(hspace=0.00)
fig.subplots_adjust(wspace=0.05)
# plot tss only plot
sns.heatmap(tss_df, cmap='magma', ax=tss_ax, cbar=False)
tss_ax.set_yticklabels(tss_ax.get_yticklabels(), rotation=0)
tss_ax.set_ylabel('')
tss_ax.set_xlabel('')
# plot sample labels
tss_colorbar_ax = fig.add_subplot((n_tss+1)*2,1,((n_tss+1)*2)-1)
sns.heatmap(mini_obs, cmap=cat_cmap,
ax=tss_colorbar_ax, cbar=False)
tss_colorbar_ax.set_ylabel('')
tss_colorbar_ax.set_xlabel('')
tss_colorbar_ax.tick_params(left=False, labelleft=False, rotation=0)
tss_colorbar_ax.tick_params(right=False, labelright=False, rotation=0)
tss_colorbar_ax.set_xticklabels('')
# plot colorbars
tss_colorbar_ax = fig.add_subplot((n_tss+1)*5,1,((n_tss+1)*5)-1)
cmap = plt.get_cmap('magma')
norm = mpl.colors.Normalize(vmin=0, vmax=1)
cb = mpl.colorbar.ColorbarBase(tss_colorbar_ax, cmap=cmap,
norm=norm, orientation='horizontal')
cb.set_label('Proportion TSS usage')
fname = '{}_{}_heatmap.pdf'.format(opref, gname)
plt.savefig(fname, dpi=300, bbox_inches='tight')
def get_cat_cmap(sample_order):
# get condition and cluster colors
samp_cdict, _ = get_sample_colors()
samples = sample_order
data = np.transpose([samples])
df = pd.DataFrame(data=data,
columns=['sample'])
# assign arbitrary numbers to each category (cluster, condition)
cats = samples
cat_dict = dict([(cat, i) for i, cat in enumerate(cats)])
df['sample'] = df['sample'].map(cat_dict)
df = df.transpose()
colors = [samp_cdict[cat] for cat in cats]
cat_cmap = ListedColormap(colors)
return df, cat_cmap
TSS switching
ab_file = 'pgp1_tss_talon_abundance.tsv'
cond_map = {'Astrocytes': ['astro_1', 'astro_2'], \
'Excitatory neurons': ['excite_neuron_1', 'excite_neuron_2'], \
'PGP1': ['pgp1_1', 'pgp1_2']}
df = pd.read_csv(ab_file, sep='\t')a
adata = make_adata(df, cond_map, how='tss')
fname = 'tss.h5ad'
adata.write(fname)
# do one test for each pair of conditions
tested = []
conditions = ['Astrocytes', 'Excitatory neurons', 'PGP1']
how = 'tss'
p = 0.05 # adjusted p-value threshold
dpi = 10 # change in percent usage (dpi) threshold
for c1 in conditions:
for c2 in conditions:
if (c1, c2) in tested or c1 == c2 or (c2, c1) in tested:
continue
else:
tested.append((c1,c2))
df = get_die(adata, [c1, c2], how=how, rc=10)
# all results
fname = '{}_{}_{}_die.tsv'.format(c1, c2, how)
df.to_csv(fname, sep='\t', index=False)
# significant results
df = filter_die_results(df, p, dpi)
fname = '{}_{}_{}_sig_die.tsv'.format(c1, c2, how)
df.to_csv(fname, sep='\t')
# visualize some TSS switches that were called
adata = sc.read('tss.h5ad')
groups = ['pgp1_1', 'pgp1_2', 'excite_neuron_1', 'excite_neuron_2', 'astro_1', 'astro_2']
tss_df = plot_tss_heatmap(adata, groups, groups, 'TCF4', 'figures/tss')
adata = sc.read('tss.h5ad')
tss_df = plot_tss_heatmap(adata, groups, groups, 'CALD1', 'figures/tss')
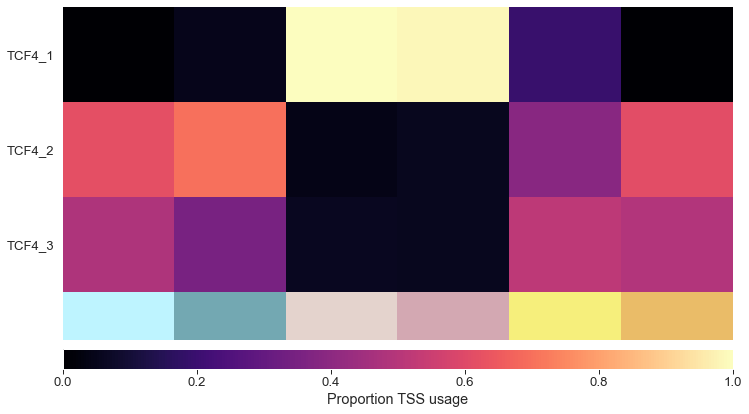
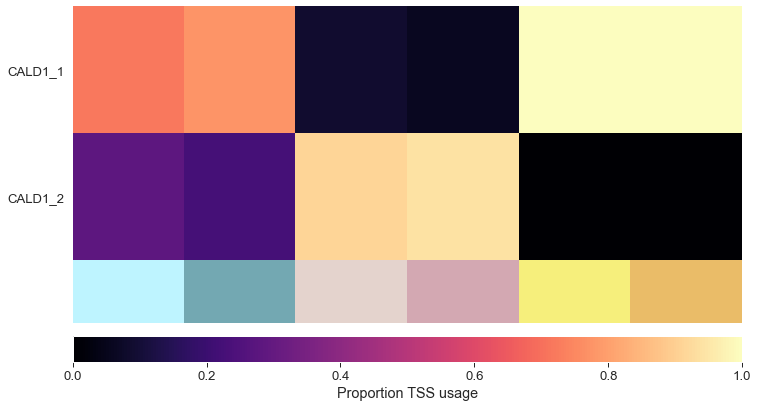
Isoform switching
ab_file = 'pgp1_tss_talon_abundance.tsv'
pass_list = 'pgp1_pass_list.csv'
cond_map = {'Astrocytes': ['astro_1', 'astro_2'], \
'Excitatory neurons': ['excite_neuron_1', 'excite_neuron_2'], \
'PGP1': ['pgp1_1', 'pgp1_2']}
df = pd.read_csv(ab_file, sep='\t')
adata = make_adata(df, cond_map, how='tss')
fname = 'tss.h5ad'
adata.write(fname)
# do one test for each pair of conditions
tested = []
conditions = ['Astrocytes', 'Excitatory neurons', 'PGP1']
how = 'tss'
p = 0.05 # adjusted p-value threshold
dpi = 10 # change in percent usage (dpi) threshold
for c1 in conditions:
for c2 in conditions:
if (c1, c2) in tested or c1 == c2 or (c2, c1) in tested:
continue
else:
tested.append((c1,c2))
print('Testing {} vs. {}'.format(c1, c2))
df = get_die(adata, [c1, c2], how=how, rc=10)
# all results
fname = '{}_{}_{}_die.tsv'.format(c1, c2, how)
df.to_csv(fname, sep='\t', index=False)
# significant results
df = filter_die_results(df, p, dpi)
fname = '{}_{}_{}_sig_die.tsv'.format(c1, c2, how)
df.to_csv(fname, sep='\t')
# use Swan to visualize some isoform switches
sg = swan.SwanGraph('swan.p')
gnames = ['TPD52L2', 'AP2M1', 'SRSF3', 'SRSF7']
for gname in gnames:
# dpi swangraph
sg.gen_report(gname, prefix='figures/{}_dpi'.format(gname),
heatmap=True,
dpi=True,
cmap='magma',
indicate_novel=True,
novelty=True)
# tpm swangraph
sg.gen_report(gname, prefix='figures/{}'.format(gname),
heatmap=True,
cmap='viridis',
indicate_novel=True,
novelty=True)
# dpi browser
sg.gen_report(gname, prefix='figures/{}_dpi'.format(gname),
heatmap=True,
dpi=True,
cmap='magma',
browser=True,
novelty=True)
# tpm browser
sg.gen_report(gname, prefix='figures/{}'.format(gname),
heatmap=True,
cmap='viridis',
browser=True,
novelty=True)
Graph from swan.p loaded
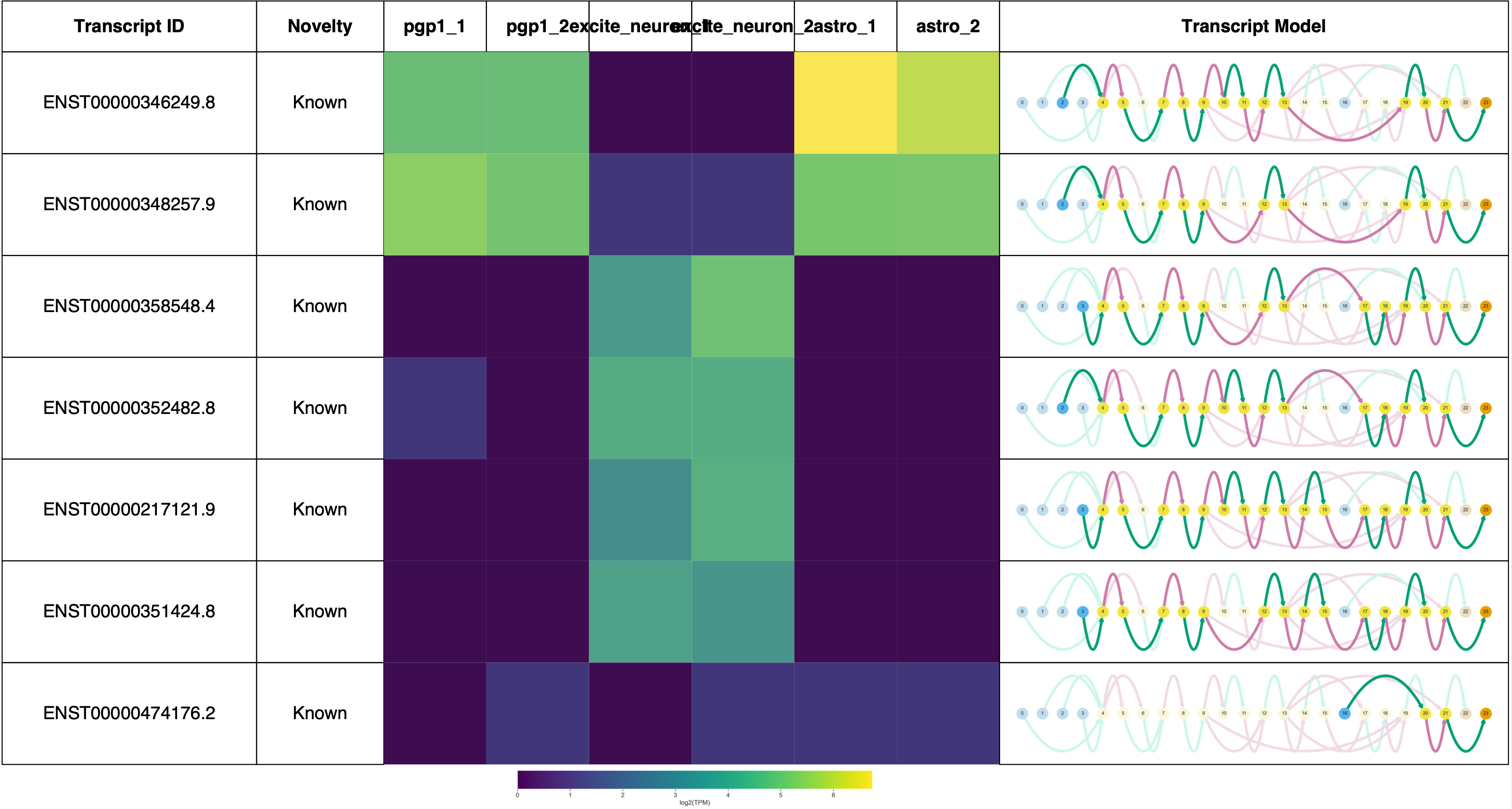
Plotting transcripts for ENSG00000101150.17
Saving transcript path graph for ENST00000346249.8 as figures/TPD52L2_dpi_novel_ENST00000346249.8_path.png
Saving transcript path graph for ENST00000348257.9 as figures/TPD52L2_dpi_novel_ENST00000348257.9_path.png
Saving transcript path graph for ENST00000358548.4 as figures/TPD52L2_dpi_novel_ENST00000358548.4_path.png
Saving transcript path graph for ENST00000352482.8 as figures/TPD52L2_dpi_novel_ENST00000352482.8_path.png
Saving transcript path graph for ENST00000217121.9 as figures/TPD52L2_dpi_novel_ENST00000217121.9_path.png
Saving transcript path graph for ENST00000351424.8 as figures/TPD52L2_dpi_novel_ENST00000351424.8_path.png
Saving transcript path graph for ENST00000474176.2 as figures/TPD52L2_dpi_novel_ENST00000474176.2_path.png
Generating report for ENSG00000101150.17
Plotting transcripts for ENSG00000101150.17
Saving transcript path graph for ENST00000346249.8 as figures/TPD52L2_novel_ENST00000346249.8_path.png
Saving transcript path graph for ENST00000348257.9 as figures/TPD52L2_novel_ENST00000348257.9_path.png
Saving transcript path graph for ENST00000358548.4 as figures/TPD52L2_novel_ENST00000358548.4_path.png
Saving transcript path graph for ENST00000352482.8 as figures/TPD52L2_novel_ENST00000352482.8_path.png
Saving transcript path graph for ENST00000217121.9 as figures/TPD52L2_novel_ENST00000217121.9_path.png
Saving transcript path graph for ENST00000351424.8 as figures/TPD52L2_novel_ENST00000351424.8_path.png
Saving transcript path graph for ENST00000474176.2 as figures/TPD52L2_novel_ENST00000474176.2_path.png
Generating report for ENSG00000101150.17
Plotting transcripts for ENSG00000101150.17
Saving transcript path graph for ENST00000346249.8 as figures/TPD52L2_dpi_browser_ENST00000346249.8_path.png
Saving transcript path graph for ENST00000348257.9 as figures/TPD52L2_dpi_browser_ENST00000348257.9_path.png
Saving transcript path graph for ENST00000358548.4 as figures/TPD52L2_dpi_browser_ENST00000358548.4_path.png
Saving transcript path graph for ENST00000352482.8 as figures/TPD52L2_dpi_browser_ENST00000352482.8_path.png
Saving transcript path graph for ENST00000217121.9 as figures/TPD52L2_dpi_browser_ENST00000217121.9_path.png
Saving transcript path graph for ENST00000351424.8 as figures/TPD52L2_dpi_browser_ENST00000351424.8_path.png
Saving transcript path graph for ENST00000474176.2 as figures/TPD52L2_dpi_browser_ENST00000474176.2_path.png
Generating report for ENSG00000101150.17
Plotting transcripts for ENSG00000101150.17
Saving transcript path graph for ENST00000346249.8 as figures/TPD52L2_browser_ENST00000346249.8_path.png
Saving transcript path graph for ENST00000348257.9 as figures/TPD52L2_browser_ENST00000348257.9_path.png
Saving transcript path graph for ENST00000358548.4 as figures/TPD52L2_browser_ENST00000358548.4_path.png
Saving transcript path graph for ENST00000352482.8 as figures/TPD52L2_browser_ENST00000352482.8_path.png
Saving transcript path graph for ENST00000217121.9 as figures/TPD52L2_browser_ENST00000217121.9_path.png
Saving transcript path graph for ENST00000351424.8 as figures/TPD52L2_browser_ENST00000351424.8_path.png
Saving transcript path graph for ENST00000474176.2 as figures/TPD52L2_browser_ENST00000474176.2_path.png
Generating report for ENSG00000101150.17
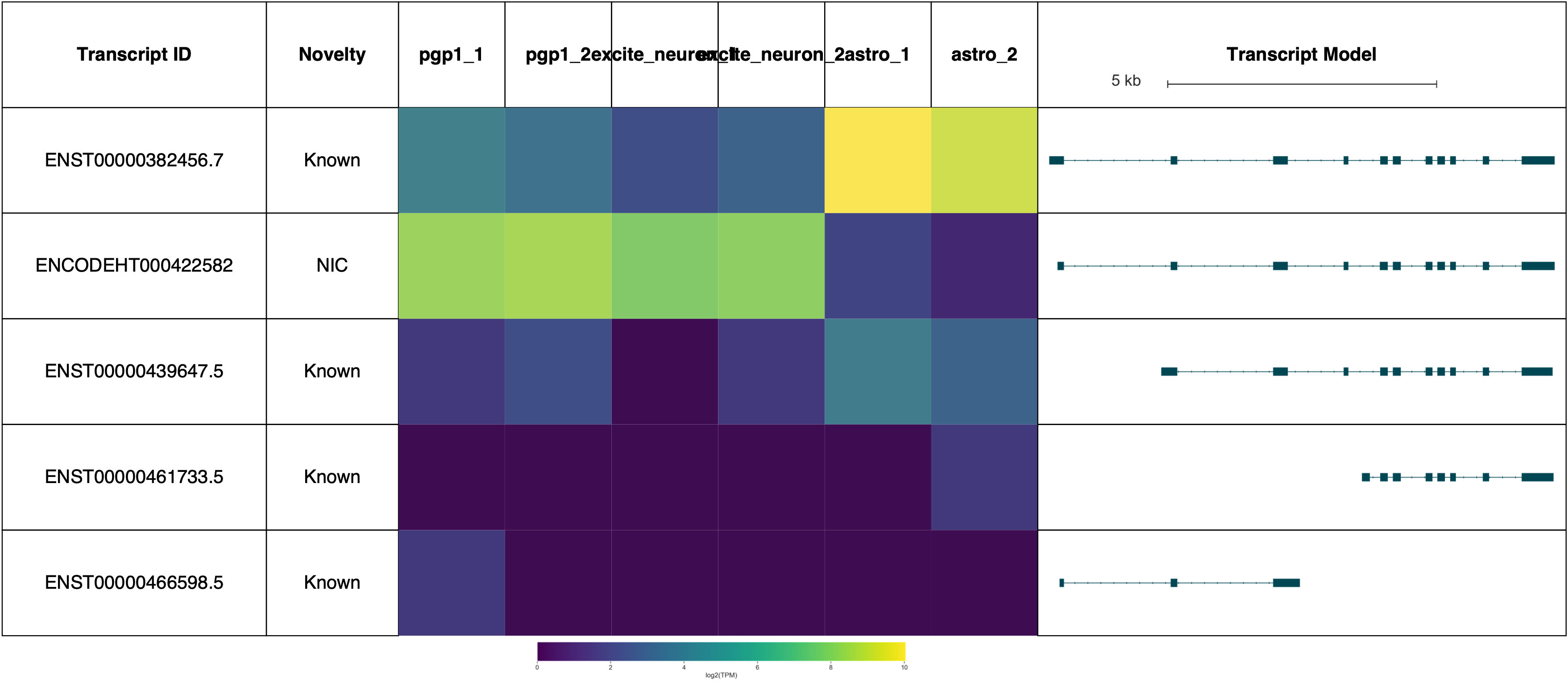
Plotting transcripts for ENSG00000161203.13
Saving transcript path graph for ENST00000382456.7 as figures/AP2M1_dpi_novel_ENST00000382456.7_path.png
Saving transcript path graph for ENCODEHT000422582 as figures/AP2M1_dpi_novel_ENCODEHT000422582_path.png
Saving transcript path graph for ENST00000439647.5 as figures/AP2M1_dpi_novel_ENST00000439647.5_path.png
Saving transcript path graph for ENST00000461733.5 as figures/AP2M1_dpi_novel_ENST00000461733.5_path.png
Saving transcript path graph for ENST00000466598.5 as figures/AP2M1_dpi_novel_ENST00000466598.5_path.png
Generating report for ENSG00000161203.13
Plotting transcripts for ENSG00000161203.13
Saving transcript path graph for ENST00000382456.7 as figures/AP2M1_novel_ENST00000382456.7_path.png
Saving transcript path graph for ENCODEHT000422582 as figures/AP2M1_novel_ENCODEHT000422582_path.png
Saving transcript path graph for ENST00000439647.5 as figures/AP2M1_novel_ENST00000439647.5_path.png
Saving transcript path graph for ENST00000461733.5 as figures/AP2M1_novel_ENST00000461733.5_path.png
Saving transcript path graph for ENST00000466598.5 as figures/AP2M1_novel_ENST00000466598.5_path.png
Generating report for ENSG00000161203.13
Plotting transcripts for ENSG00000161203.13
Saving transcript path graph for ENST00000382456.7 as figures/AP2M1_dpi_browser_ENST00000382456.7_path.png
Saving transcript path graph for ENCODEHT000422582 as figures/AP2M1_dpi_browser_ENCODEHT000422582_path.png
Saving transcript path graph for ENST00000439647.5 as figures/AP2M1_dpi_browser_ENST00000439647.5_path.png
Saving transcript path graph for ENST00000461733.5 as figures/AP2M1_dpi_browser_ENST00000461733.5_path.png
Saving transcript path graph for ENST00000466598.5 as figures/AP2M1_dpi_browser_ENST00000466598.5_path.png
Generating report for ENSG00000161203.13
Plotting transcripts for ENSG00000161203.13
Saving transcript path graph for ENST00000382456.7 as figures/AP2M1_browser_ENST00000382456.7_path.png
Saving transcript path graph for ENCODEHT000422582 as figures/AP2M1_browser_ENCODEHT000422582_path.png
Saving transcript path graph for ENST00000439647.5 as figures/AP2M1_browser_ENST00000439647.5_path.png
Saving transcript path graph for ENST00000461733.5 as figures/AP2M1_browser_ENST00000461733.5_path.png
Saving transcript path graph for ENST00000466598.5 as figures/AP2M1_browser_ENST00000466598.5_path.png
Generating report for ENSG00000161203.13
Plotting transcripts for ENSG00000112081.16
Saving transcript path graph for ENST00000373715.10 as figures/SRSF3_dpi_novel_ENST00000373715.10_path.png
Saving transcript path graph for ENST00000477442.6 as figures/SRSF3_dpi_novel_ENST00000477442.6_path.png
Saving transcript path graph for ENST00000620389.1 as figures/SRSF3_dpi_novel_ENST00000620389.1_path.png
Saving transcript path graph for ENST00000614136.1 as figures/SRSF3_dpi_novel_ENST00000614136.1_path.png
Saving transcript path graph for ENST00000339436.11 as figures/SRSF3_dpi_novel_ENST00000339436.11_path.png
Generating report for ENSG00000112081.16
Plotting transcripts for ENSG00000112081.16
Saving transcript path graph for ENST00000373715.10 as figures/SRSF3_novel_ENST00000373715.10_path.png
Saving transcript path graph for ENST00000477442.6 as figures/SRSF3_novel_ENST00000477442.6_path.png
Saving transcript path graph for ENST00000620389.1 as figures/SRSF3_novel_ENST00000620389.1_path.png
Saving transcript path graph for ENST00000614136.1 as figures/SRSF3_novel_ENST00000614136.1_path.png
Saving transcript path graph for ENST00000339436.11 as figures/SRSF3_novel_ENST00000339436.11_path.png
Generating report for ENSG00000112081.16
Plotting transcripts for ENSG00000112081.16
Saving transcript path graph for ENST00000373715.10 as figures/SRSF3_dpi_browser_ENST00000373715.10_path.png
Saving transcript path graph for ENST00000477442.6 as figures/SRSF3_dpi_browser_ENST00000477442.6_path.png
Saving transcript path graph for ENST00000620389.1 as figures/SRSF3_dpi_browser_ENST00000620389.1_path.png
Saving transcript path graph for ENST00000614136.1 as figures/SRSF3_dpi_browser_ENST00000614136.1_path.png
Saving transcript path graph for ENST00000339436.11 as figures/SRSF3_dpi_browser_ENST00000339436.11_path.png
Generating report for ENSG00000112081.16
Plotting transcripts for ENSG00000112081.16
Saving transcript path graph for ENST00000373715.10 as figures/SRSF3_browser_ENST00000373715.10_path.png
Saving transcript path graph for ENST00000477442.6 as figures/SRSF3_browser_ENST00000477442.6_path.png
Saving transcript path graph for ENST00000620389.1 as figures/SRSF3_browser_ENST00000620389.1_path.png
Saving transcript path graph for ENST00000614136.1 as figures/SRSF3_browser_ENST00000614136.1_path.png
Saving transcript path graph for ENST00000339436.11 as figures/SRSF3_browser_ENST00000339436.11_path.png
Generating report for ENSG00000112081.16
Plotting transcripts for ENSG00000115875.18
Saving transcript path graph for ENST00000313117.10 as figures/SRSF7_dpi_novel_ENST00000313117.10_path.png
Saving transcript path graph for ENST00000446327.6 as figures/SRSF7_dpi_novel_ENST00000446327.6_path.png
Saving transcript path graph for ENST00000443213.5 as figures/SRSF7_dpi_novel_ENST00000443213.5_path.png
Saving transcript path graph for ENST00000409276.5 as figures/SRSF7_dpi_novel_ENST00000409276.5_path.png
Saving transcript path graph for ENST00000425778.5 as figures/SRSF7_dpi_novel_ENST00000425778.5_path.png
Saving transcript path graph for ENST00000431066.5 as figures/SRSF7_dpi_novel_ENST00000431066.5_path.png
Saving transcript path graph for ENST00000477635.5 as figures/SRSF7_dpi_novel_ENST00000477635.5_path.png
Generating report for ENSG00000115875.18
Plotting transcripts for ENSG00000115875.18
Saving transcript path graph for ENST00000313117.10 as figures/SRSF7_novel_ENST00000313117.10_path.png
Saving transcript path graph for ENST00000446327.6 as figures/SRSF7_novel_ENST00000446327.6_path.png
Saving transcript path graph for ENST00000443213.5 as figures/SRSF7_novel_ENST00000443213.5_path.png
Saving transcript path graph for ENST00000409276.5 as figures/SRSF7_novel_ENST00000409276.5_path.png
Saving transcript path graph for ENST00000425778.5 as figures/SRSF7_novel_ENST00000425778.5_path.png
Saving transcript path graph for ENST00000431066.5 as figures/SRSF7_novel_ENST00000431066.5_path.png
Saving transcript path graph for ENST00000477635.5 as figures/SRSF7_novel_ENST00000477635.5_path.png
Generating report for ENSG00000115875.18
Plotting transcripts for ENSG00000115875.18
Saving transcript path graph for ENST00000313117.10 as figures/SRSF7_dpi_browser_ENST00000313117.10_path.png
Saving transcript path graph for ENST00000446327.6 as figures/SRSF7_dpi_browser_ENST00000446327.6_path.png
Saving transcript path graph for ENST00000443213.5 as figures/SRSF7_dpi_browser_ENST00000443213.5_path.png
Saving transcript path graph for ENST00000409276.5 as figures/SRSF7_dpi_browser_ENST00000409276.5_path.png
Saving transcript path graph for ENST00000425778.5 as figures/SRSF7_dpi_browser_ENST00000425778.5_path.png
Saving transcript path graph for ENST00000431066.5 as figures/SRSF7_dpi_browser_ENST00000431066.5_path.png
Saving transcript path graph for ENST00000477635.5 as figures/SRSF7_dpi_browser_ENST00000477635.5_path.png
Generating report for ENSG00000115875.18
Plotting transcripts for ENSG00000115875.18
Saving transcript path graph for ENST00000313117.10 as figures/SRSF7_browser_ENST00000313117.10_path.png
Saving transcript path graph for ENST00000446327.6 as figures/SRSF7_browser_ENST00000446327.6_path.png
Saving transcript path graph for ENST00000443213.5 as figures/SRSF7_browser_ENST00000443213.5_path.png
Saving transcript path graph for ENST00000409276.5 as figures/SRSF7_browser_ENST00000409276.5_path.png
Saving transcript path graph for ENST00000425778.5 as figures/SRSF7_browser_ENST00000425778.5_path.png
Saving transcript path graph for ENST00000431066.5 as figures/SRSF7_browser_ENST00000431066.5_path.png
Saving transcript path graph for ENST00000477635.5 as figures/SRSF7_browser_ENST00000477635.5_path.png
Generating report for ENSG00000115875.18
TPD52L2 reports: (Forgive the terrible sample labels, this will be fixed in a future Swan release!)
AP2M1 reports: (Forgive the terrible sample labels, this will be fixed in a future Swan release!)
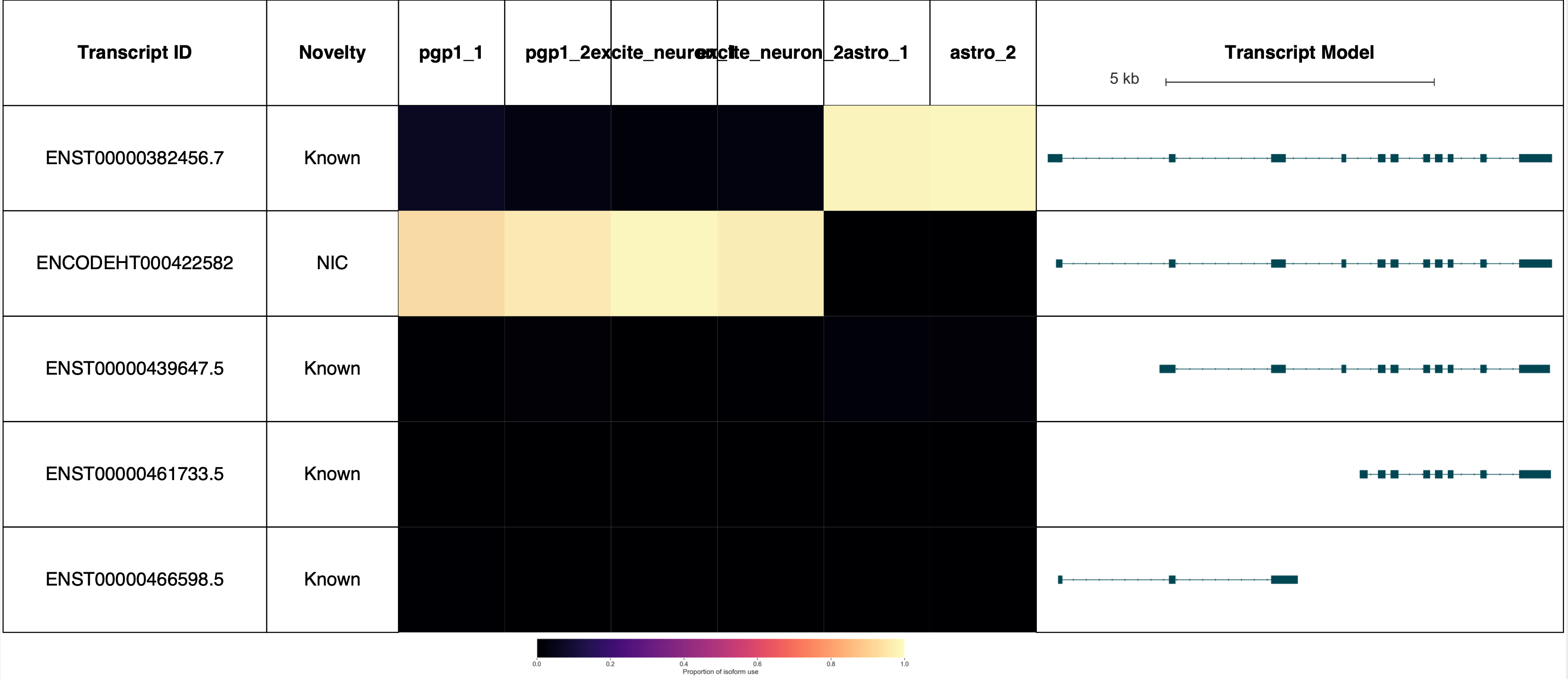
Visualizing reads and TSSs on the genome browser
Hub file
Use this link to view my tracks on your genome browser session
Convert TSS bed to bigBed
Convert the bed file of filtered TSSs that can be displayed on the genome browser.
To download the bedToBigBed software, use the conda installation instructions here: https://anaconda.org/bioconda/ucsc-bedtobigbed
bed='filt_tss.bed'
chrom_sizes='hg38.chrom.sizes'
bedToBigBed \
$bed \
$chrom_sizes \
filt_tss.bb
Creating and hosting the track hub
This part requires you have use of a server with public-facing access example. The old HPC had one which was really useful (the link used to look like https://hpc.oit.uci.edu/~freese), but there is none for HPC3 and they are not planning on releasing one (I asked HPC support about this). Therefore I’m not sure how useful this will be for those that do not have their own lab server.
c_dict, order = get_sample_colors()
sample_file = 'samples.txt' # sample file with one sample per line
genome = 'hg38' # genome
hub_name = 'pgp1' # name of hub
tss = 'filt_tss.bb' # tss file
email = 'freese@uci.edu' # email of hub creator
# URL location of hub
url = 'http://crick.bio.uci.edu/freese/210413_pgp1_hub/'
# ssh location of hub (used to copy)
# if you do not want to automatically move files to server, use the
# commented-out version of the `make_hub()` call.
scp_location = 'freese@crick.bio.uci.edu:~/pub/210413_pgp1_hub/'
make_hub(url, c_dict, samples, genome, hub_name, email, scp_location=scp_location)
# make_hub(url, c_dict, samples, genome, hub_name, email, scp_location=None)
def make_bam_hub_entry(df, c_dict, ofile):
with open(ofile, 'w') as o:
for ind, e in df.iterrows():
c = c_dict[e['sample']]
c = c.lstrip('#')
c = tuple(int(c[i:i+2], 16) for i in (0, 2, 4))
s = 'track {}_reads\n'.format(e['sample'])
s += 'bigDataUrl {}\n'.format(e.url)
s += 'shortLabel {}_reads\n'.format(e['sample'])
s += 'longLabel {}_reads\n'.format(e['sample'])
s += 'type bam\n'
s += 'visibility squish\n'
s += 'bamColorMode off\n'
s += 'color {},{},{}\n\n'.format(c[0],c[1],c[2])
o.write(s)
def make_tss_hub_entry(tss, url, ofile):
with open(ofile, 'a') as o:
s = 'track tss\n'
s += 'type bigBed 6\n'
s += 'bigDataUrl {}{}\n'.format(url, tss)
s += 'shortLabel tss \n'
s += 'longLabel tss\n'
s += 'visibility dense\n'
o.write(s)
# this function probably won't be useful for anyone
# relies on talon_tmp/ bam files
def make_hub(url, c_dict, samples, genome, hub_name, email, scp_location=None):
genomefile = 'hub/genomes.txt'
hubfile = 'hub/hub.txt'
hubfile_relative = 'hub.txt'
trackdb = 'hub/{}/trackDb.txt'.format(genome)
relative_trackdb = '{}/trackDb.txt'.format(genome)
relative_genome = 'genomes.txt'
try:
os.makedirs(os.path.dirname(trackdb))
except:
pass
df = pd.read_csv(sample, header=None, names=['sample'])
df['url'] = df.apply(lambda x: url+x['sample']+'.bam', axis=1)
df['local_loc'] = df.apply(lambda x: 'talon_tmp/'+x['sample']+'.bam', axis=1)
for ind, entry in df.iterrows():
cmd = 'samtools index {}'.format(entry.local_loc)
print(cmd)
cmd = shlex.split(cmd)
result = subprocess.run(cmd)
if scp_location:
cmd = 'scp {} {}'.format(entry.local_loc, scp_location)
cmd = shlex.split(cmd)
print(cmd)
result = subprocess.run(cmd)
cmd = 'scp {}.bai {}'.format(entry.local_loc, scp_location)
cmd = shlex.split(cmd)
print(cmd)
result = subprocess.run(cmd)
make_bam_hub_entry(df, c_dict, trackdb)
make_tss_hub_entry(tss, url, trackdb)
with open(genomefile, 'w') as o:
s = 'genome {}\n'.format(genome)
s += 'trackDb {}\n'.format(relative_trackdb)
o.write(s)
with open(hubfile, 'w') as o:
s = 'hub {}\n'.format(hub_name)
s += 'shortLabel {}\n'.format(hub_name)
s += 'longLabel {}\n'.format(hub_name)
s += 'genomesFile {}\n'.format(relative_genome)
s += 'email {}\n'.format(email)
o.write(s)
if scp_location:
cmd = 'scp -r hub/ {}'.format(scp_location)
cmd = shlex.split(cmd)
result = subprocess.run(cmd)
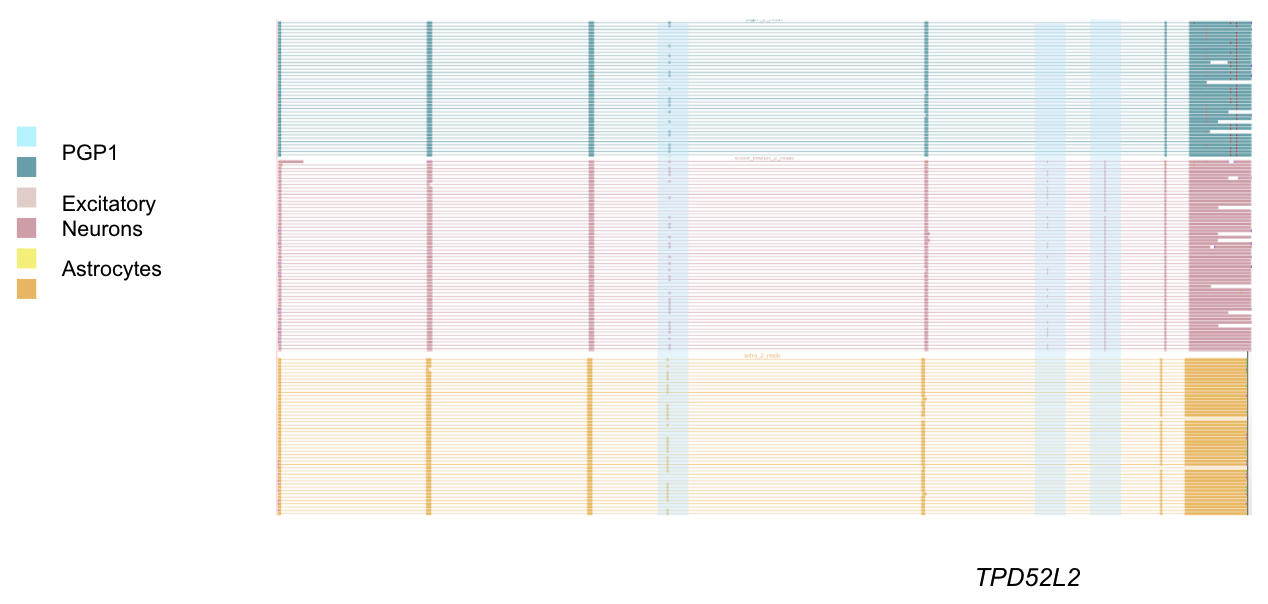
A (cropped) view of the TPD52L2 locus on the track hub, with TSSs in black, astrocyte reads in orange, PGP1 reads in blue, and excitatory neuron reads in pink. Blue highlights (added afterward) show locations of alternative exons